Intro to Lean CS
Lean CS meets Customer expectations while using minimal resources, meeting regulatory requirements, and engaging staff through continuous improvement.
Once a month, be on the lookout for the Process Pro’s tips on successfully implementing and sustaining lean principles in the sterile processing environment. While entire books are written on lean, we will be focusing on a simple principle each month to understand and incorporate into your department.
Try Googling “definition of Lean.” You won’t find one agreed-upon definition. So, here’s a sterile processing version that we can use:
Lean CS meets Customer expectations while using minimal resources, meeting regulatory requirements, and engaging staff through continuous improvement.
This means:
 We must measure our Customer’s expectations by collecting data on complete and sterile trays, complete case carts, on-time delivery, etc.
We must measure our Customer’s expectations by collecting data on complete and sterile trays, complete case carts, on-time delivery, etc.- We need to measure our resource usage against a lean standard, such as labor-hours worked versus trays processed.
- We must ensure we’re in compliance with regulatory requirements.
- We need to develop an environment where our staff is engaged, the department is continuously improving, and we’re measuring our performance.
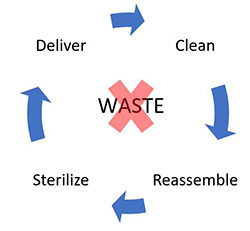
Another simple way I like to introduce Lean CS is to focus on the core activity every CS: Processing instruments. Our Customers send us used instruments with the expectation that we process and return them in a timely manner. To do this, in very simple terms, we clean, reassemble, sterilize and deliver the instruments. This is the value we provide.
Now for the fun part! Anything you see in your department other than cleaning, assembling, sterilizing, and delivering instruments is waste and not Lean CS! Moving instrument trays from the washer to a staging rack, waste! Staff’s excess walking, waste! Instruments waiting to be worked on, waste! Looking for lost instruments, waste! Though the waste may not be our fault, much of it can be minimized or eliminated completely. For example, some claim that looking for a missing instrument is just “part of assembly,” but the instrument shouldn’t have been lost in the first place and highlights a process breakdown.
Lean CS is more than how we do the work; it’s how we manage the work. How we do the work is “Lean Production” and focuses on continuously improving quality and efficiency while meeting Customer requirements. How we manage the work is “Lean Management” and focuses on the tools and leadership routines that sustain lean production, expose waste and operational issues, and engages staff in continuous improvement.
I hope this introduction helps stimulate your thinking of Lean CS principles. Stay tuned for more Lean CS tips from the Process Pro’s next month.